Abstract
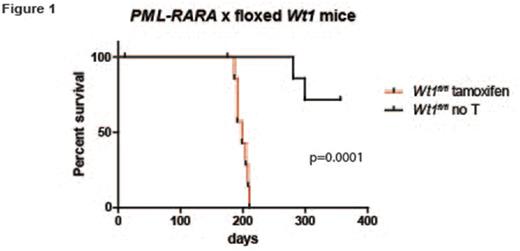
Inactivating mutations in Wilms Tumor 1 (WT1) are found in 5-10% of cases of acute myeloid leukemia (AML). Paradoxically, wild type WT1 is expressed highly in most cases of AML compared to normal hematopoietic progenitors, and high levels of WT1 have been associated with poor outcomes. Therefore, it is not yet clear whether loss-of-function WT1 mutations-or paradoxically, wild type WT1 overexpression-both promote disease progression in AML. We performed a genome-wide screen for mutations in acute promyelocytic leukemia (APL), a morphologic subtype of AML characterized by the PML-RARA fusion. We identified WT1 mutations in 11/42 patients, suggesting that WT1 mutations may cooperate specifically with PML-RARA in APL. Further, expression analysis demonstrated a high level of wild type WT1 expression in non-mutated cases. To test the effect of Wt1 inactivation in a mouse model of APL, we bred mice carrying a floxed Wt1 allele (give Baylor reference) to transgenic mice expressing tamoxifen-inducible Cre recombinase (ERT2-Cre). Tamoxifen treatment leads to deletion of the C-terminal zinc finger domains of Wt1, mimicking some of the mutations that are commonly found in AML/APL cases. These mice were crossed with mice expressing the PML-RARA fusion cDNA in early myeloid progenitors with Cathepsin G (Ctsg) regulatory sequences (Ctsg-PML-RARA). Bone marrow cells from Ctsg-PML-RARA x Wt1fl/fl x ERT2-Cre mice were transplanted into irradiated recipients. Tamoxifen-treated mice (i.e. Wt1 deficient) developed APL with a significantly shortened latency compared to untreated controls (198 days vs. endpoint not reached, N=8-10 each group, p=.0001), suggesting that Wt1 loss-of-function mutations can cooperate with PML-RARA to drive APL. Next, to test the effect of wild type Wt1 overexpression on hematopoietic cells, bone marrow cells from wild type or Ctsg-PML-RARA mice was transduced with retroviruses expressing full length Wt1 cDNA or an empty vector control, and subjected to serial replating. As previously reported, untransduced bone marrow cells from Ctsg-PML-RARA mice were able to form colonies in methylcellulose week after week, while cells overexpressing wild type Wt1 were selected against during serial replating, as demonstrated by a gradual reduction in GFP percentage and Wt1 expression, as measured by western blotting. Interestingly, in contrast to human AML/APL, Wt1 expression could not be detected in a panel of murine tumors with initiating mutations in DNMT3A, PML-RARA, or MLL-AF9 . Taken together, these findings suggest that Wt1 expression suppresses APL development in mice.
To reconcile these findings with the high level of WT1 expression in human AML/APL, we speculated that in human progenitor cells, WT1 expression may be induced by leukemia-associated oncogenes (like PML-RARA) as a physiologic, inhibitory response to oncogenic stress. In this case, subsequent inactivating mutations in WT1 would provide an additional growth advantage. Given the observed interspecies difference between WT1/Wt1 expression levels in mouse and human leukemia cells, we used human umbilical cord blood-derived CD34+ cells transduced with retroviruses expressing PML-RARA to model early events in human APL development. CD34+ cells expressing PML-RARA share some characteristics of fully transformed APL cells, including enhanced proliferation in liquid culture, and reduced expression of the myeloid maturation markers CD11b and CD14. WT1 mRNA expression is low in freshly isolated CD34+ cells, but rapidly increases in cells expressing PML-RARA over two weeks in culture (10.5-fold increase compared to empty-vector transduced controls, N=3 separate experiments, p<0.05). Western blotting confirmed robust induction of WT1 protein in transformed cells compared to empty-vector transduced controls, suggesting that WT1 expression is a normal consequence of PML-RARA activation in human hematopoietic progenitor cells. In summary, these findings support a cooperating role for WT1 mutations in APL development, and a potential mechanism to explain the high level of wild type WT1 expression observed in APL cells. Further experiments will be required to determine the downstream mechanisms by which WT1 antagonizes APL development, and whether these observations are generalizable to other AML subtypes.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal